Introduction. Total knee replacement (TKR) and total hip replacement (THA) surgeries have historically been felt to carry a high risk of post-operative venous thromboembolism (VTE) events thereby warranting anticoagulant prophylaxis. This risk however was based on composite endpoints in which asymptomatic DVT on venography, as opposed to symptomatic VTE or VTE-related mortality, constituted the majority of the events. Furthermore, current day orthopedic practices have likely led to a reduction in risk of VTE, thought to be 3-5% in the absence of prophylaxis. While the American College of Chest Physicians (ACCP) guidelines recommend the use of an anticoagulant for prophylaxis for all patients undergoing TKR and THR over aspirin, American Academy of Orthopaedic Surgeons (AAOS) guidelines classify patients without a prior history of VTE, and those not meeting their high risk criteria (significant cardiovascular disease; BMI > 40; smoking + DM + BMI > 35; and recent cancer) as low-risk; aspirin (325 mg twice daily) is a recommended alternative for post-operative VTE prophylaxis in these patients. As a result, many orthopedic surgeons in the US use aspirin for patients without major risk factors for VTE other than the surgery itself, accounting for over 40% joint replacement surgery cases in the US. The risk of symptomatic VTE in this patient population outside of small and primarily retrospective studies remains unknown. We conducted a prospective cohort study of patients receiving aspirin thromboprophylaxis following TKR and THR surgery at a large orthopedic specialty hospital to assess the risk of clinically symptomatic VTE and bleeding events. This is a preliminary analysis of planned total accrual of 500 subjects (assuming 2% VTE event rate in aspirin-treated patients, as compared to 1% in anticoagulant-treated, with alpha and beta error levels of 5% and 50%, respectively, and accounting for attrition rate of at least 10%).
Methods. All TKR and THR patients prescribed aspirin for VTE prophylaxis by the surgical team and not on any anticoagulant medications were eligible for the study. Study subjects were identified and consented post-operatively prior to their hospital discharge. Enrolled subjects were followed for symptomatic VTE and bleeding events during their hospitalization, and then contacted at 30 and 90 days postoperatively for a telephone survey. Symptomatic VTE and bleeding events were captured using a questionnaire and outside records obtained to confirm VTE or major bleeding events. Three-month risk of symptomatic VTE and major bleeding events associated with aspirin thromboprophylaxis were estimated.
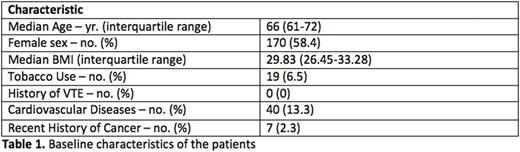
Results. A total of 300 patients, 199 TKR and 101 THR, have been enrolled in this ongoing study so far. Table 1 shows the baseline characteristics of the patients. A total of 6 symptomatic VTE (4 pulmonary emboli, 1 proximal deep vein thrombosis (DVT) and 1 isolated-distal DVT) occurred during the follow up period. (3-month post-op VTE event rate of 2%). 3 events were diagnosed during hospitalization, whereas the remaining 3 occurred following hospital discharge (within 7-10 days). Bruising was a common side effect affecting nearly 3% of the patients, but major bleeding events were rare, only 1 major upper gastrointestinal bleeding, giving a 3-month risk of 0.33%. There were no surgical bleeds reported.
Conclusions. In this single-center prospective cohort study of TKR and THR patients considered to be at lower risk for VTE, the use of aspirin for thromboprophylaxis was associated with a 2% symptomatic VTE event rate over three months of follow up with minimal bleeding risk. This result, together with those of the EPCATII study from Canada showing that low-dose aspirin was as effective (~0.7% event rate) following 5 days of rivaroxaban following TKR and THR in lower risk patients, argues for a large randomized trial evaluating aspirin as monotherapy for VTE prophylaxis in such patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.